Covid-19 vaccine in the US news summary: 12 May 2021
The latest US vaccine news and global developments, as everyone 16 and over is eligible for the coronavirus vaccine in every US state. Wednesday 12 May.

Show key events only
Covid-19 vaccine latest news | Wed 12 May
Can covid-19 vaccines cause a rash? What to do if you have an allergic reaction
As the vaccine rollout continues, some recipients have reported experiencing a red, itchy, swollen or painful rash around the area where they got the shot, which can appear a week after the injection was received.
These rashes are sometimes known as ‘covid arm’ according to the Centers for Disease Control and Prevention (CDC) but they advise that it should not stop you getting the second dose, unless there are further symptoms. Here's what the CDC say about the rash and other allergic reactions to covid-19 vaccines.
Children are now eligible to receive a covid-19 vaccine
The CDC has altered its recommendation on vaccination procedures to expand eligibility for the Pfizer/BioNTech vaccine to all children aged 12-15 for the first time. Children are less likely to suffer severe effects if they become infected with the virus so have been excluded up to this point, but having now administered nearly 250 million doses the US is able to open up eligibility even further.
Covid-19 vaccines: what does 95% efficacy mean?
The ongoing vaccination effort is one of the most important parts of President Biden's plan to combat the pandemic, and the United States is set to surpass an important milestone in the coming hours.
So far three covid-19 vaccines have been approved for emergency use in the US by the Food and Drug Administration and eligibility has recently been extended to all American adults. One of the most talked about differences between the three vaccines is their efficacy, but ranking them may not be as easy as it appears.
Vaccine lottery will pay out $1 million a week
Ohio Governor Mike DeWine has confirmed details of the state's upcoming 'vaccine lottery', which will see recipients of the life-saving shot entered into a weekly prize draw worth $1 million. In the announcement, he said: "the real waste at this point in the pandemic - when the vaccine is readily available to anyone who wants it - is a life lost to covid-19."
Many states are offering some form of incentive to residents to get the vaccine as they attempt to reach herd immunity as quickly as possible. West Virginia is offering $100 savings bonds to those aged 16-35 who get vaccinated, while breweries participating in New Jersey’s “Shot and a Beer” scheme are giving out free drinks.
US on the verge of crossing vaccine milestone
The official presidential Twitter account has confirmed that the United States is set to reach 250 million shots of covid-19 vaccines tomorrow. The milestone represents one of the swiftest vaccination efforts in the world and comes as a CDC advisory panel approves the use of Pfizer's vaccine for children aged 12-15.
The vaccine rollout is crucial to reaching a state of herd immunity which will allow the nation to return to normality, possibly sometime later this year. However as the effort continues there is some evidence that reluctancy amongst some groups to receive the vaccination could extend the pandmeic for longer than is neccessary.

35.4% of the US population now fully vaccinated
A total of 117,647,439 people in the United States, 35.4% of the population have received both shots of the Covid-19 vaccine and are now fully inoculated.
153,986,312 people have been given at least one shot - which represents 46.4% of the population.
CDC advisory panel approves Pfizer vaccine for children aged 12-15
Just in, the CDC Advisory committee has voted to endorse the Pfizer vaccine for children aged from 12-15. The vote was 14 in favour, 1 abstain/recuse, none against.
Earlier in the week, the Food and Drug Administration authorized emergency use of the two-shot vaccine made by Pfizer and its German partner, BioNTech. A study of more than 2,000 12- to 15-year-olds found the same dose adults use is safe and strongly protective in the kids, too.
More on that shortly...
US trade chief says vaccine waiver talks could make drug firms heroes
US Trade Representative Katherine Tai said on Wednesday she is pushing for a waiver of Covid-19 vaccine intellectual property rights because the United States and drug makers have "an obligation to help save the world right now."
Tai, speaking at a US Senate Finance Committee hearing, said that she views the World Trade Organization talks as a way to remove the intellectual property issue as an obstacle to vaccine production. She backed the WTO negotiations last week. She praised the work of US companies in quickly developing and producing safe and effective vaccines, adding that on intellectual property, "The message that I want to give to them is, 'You can be a hero here.''
Several Republican senators criticized Tai for 'giving away' US innovations to foreign competitors by supporting the WTO negotiations. Tai said she views the talks to be less about preventing other countries from 'stealing' U.S. technology and more about finding a way to have a positive impact on people's lives by ending the coronavirus pandemic.
"What we are trying to accomplish is the saving of lives," she said, adding that ending the pandemic is a necessary first step in any trade policy going forward. "Unless we are able to bring the rest of world's economies back online, there's not going to be a lot of upside for us in what we're going to be doing" on trade, Tai said.
Asked by Democratic Senator Elizabeth Warren whether USTR would support a broader WTO intellectual property waiver on Covid-19 treatments, therapeutics, protective equipment and other medical products, Tai said she is currently only focused on increasing vaccine access and equity.

Double world's coronavirus vaccine production, pleads UN chief
United Nations Secretary-General Antonio Guterres called on Wednesday for the need to double the capacity of Covid-19 vaccine production and for fairer redistribution of the shots in the developing world, which faces new waves of the coronavirus.
Many countries are experiencing shortages of the vaccine, especially India, worsening a dire second wave of infections that has left hospitals and morgues overflowing while families scramble for increasingly scarce medicines and oxygen. At the same time, the European Union has reserved a surplus of the vaccines.
"It is totally unacceptable to live in the world, in which developed countries can vaccinate most of its population, while many developing countries have not access to one single dose," Guterres told a briefing after meeting Russian foreign minister Sergei Lavrov in Moscow. He mentioned the risks of coronavirus mutations and new variants as the virus spreads 'like wildfire' in different parts of the developing world. "So, that's in interest of everybody that everybody is vaccinated everywhere. We believe that we need two things: to double the world's capacity of production of vaccines and at the same time to have a more equitable distribution of vaccines", Guterres said.

U.S. CDC finds more clotting cases after J&J vaccine, sees causal link
(Reuters) -The U.S. Centers for Disease Control and Prevention said on Wednesday it had found more cases of potentially life-threatening blood clotting among people who received the Johnson & Johnson COVID-19 vaccine and sees a “plausible causal association”.
The CDC said in a presentation the agency has now identified 28 cases of thrombosis with thrombocytopenia syndrome (TTS) among the more than 8.7 million people who had received the J&J vaccine. TTS involves blood clots accompanied by a low level of platelets - the cells in the blood that help it to clot.
So far, three of the 28 have died. Previously, as of April 25, the CDC had reported 17 cases of clotting among nearly 8 million people given vaccines.
The Advisory Committee on Immunization Practices or ACIP, which advises the U.S. CDC, recommended on April 23 that the U.S. lift a 10-day pause on the J&J vaccinations imposed to review safety data on the clotting issue. The panel will review the new data later on Wednesday.
The CDC said on Wednesday the events appear similar to what is being observed following administration of the AstraZeneca COVID-19 vaccine in Europe.
Both vaccines are based on a new technology using adenoviruses, which cause the common cold, that have been modified to essentially render them harmless. The viruses are used to carry instructions into the body to make specific coronavirus proteins, priming the immune system to make antibodies that fight off the actual virus.
Scientists are working to find the potential mechanism that would explain the blood clots. A leading hypothesis appears to be that the vaccines are triggering a rare immune response that could be related to these viral vectors.
The syndrome does not appear to be associated with either of the COVID-19 vaccines produce by Pfizer Inc and BioNTech SA or Moderna Inc.
Most of the cases were among women aged 18 to 49, the CDC said, with rates among women aged 30-39 at 12.4 cases per million and those aged 40-49 at 9.4 cases per million.
Only six of the clotting events identified were in men.
Symptoms typically occur several days after vaccination to up to 2 weeks.
Overcoming barriers to vaccination
Politico looks into many of the barriers to getting vaccinated as public health experts identify solutions to help limit these barriers. These issues are confronted using different strategies need to reduce hesitancy, which has less to do with the logistics of getting to a vaccine appointment and more with distrust of government and the development process. Read the full story here.
Senior Vaccination Update
According to the CDC, Vaccinations among seniors are up to eight percent since late April. On average, across the country, 72% of residents over sixty-five are fully vaccinated. Seventy-eight percent of seniors have received at least one dose of a covid-19 vaccine. Deaths and hospitalizations among seniors are also down across the country -- a prime example that vaccination does help to protect the most vulnerable.
In an interview with MSNBC, Paul Offit, a member of the FDA Vaccines and Related Biological Products Advisory Committee, explains why he believes CDC will approve the Pfizer vaccine for children between 12-15.
Morning Consult reports on what activities vaccinated and unvaccinated adults are more likely to take part in.
Major divides between the groups include "Going on a cruise," "Attending a concert," and "Traveling abroad."
Full details here.
Can kids between the ages of 12-15 get a covid-19 vaccine?
The Food and Drug Administration has approved Pfizer and BioNTech’s for use in children as young as twelve. But, before shots can begin to be administered to this group, the Center for Disease Control and Prevention (CDC), must give their recommendation, which is expected today.
In anticipation of the CDC’s approval, some states and cities are moving to let parents and guardians make appointments for their children. In Chicago, parents are able to book appointments for their children starting Thursday.
Other states and localities are awaiting guidance from the CDC to make vaccine appointments available to children.
All you need to know on this from Maite Knorr-Evans.
Vaccine patent suspensions: US and German friction
The EU recently announced that it would be focusing its effort on mRNA vaccines starting in 2022 and has ordered another 1.8 billion doses from BioNTech. Furthermore, in a moment of rare forthrightness, the Chinese government said that the vaccines developed in the country, based on traditional technologies, weren't good enough.
Chinese vaccines "don't have very high rates of protection," said Gao Fu, head of the Chinese Centers for Disease Control and Prevention. "Everyone should consider the benefits mRNA vaccines can bring for humanity," he added. Other countries, including the United States and Japan, are also planning to shift their focus partly or entirely to mRNA vaccines.
Rarely has a virtually unknown technology proven its worth so quickly. "Even I didn't expect this to happen so clearly and at this speed," says BioNTech CEO Uğur Şahin. The company is expecting to accumulate revenues of 9.8 billion euros this year, more than twice what it projected as recently as 2020. Analysts are predicting global vaccine earnings of around $100 billion or more per year.
An interesting report by Claus Hecking, Michael Sauga, Thomas Schulz and Gerald Traufetter for Der Spiegel.
New deadly strain not named 'Indian variant'
India has been battling a new covid-19 strain that has raised global concerns. However, it has been reported that the exact strain has been found in Nigeria.
The Union Health Ministry has clarified that the World Health Organisation (WHO) has not classified B.1.617 mutant of the novel coronavirus as an "Indian Variant" in its document.
It dismissed as "without any basis and unfounded" media reports that have used the term "Indian variant" for the B.1.617 mutant strain, which the WHO recently said was a "variant of global concern".
Ride for free and get vaccinated
A reminder that New Yorkers could start getting some free rides on the subway if they get vaccinated at certain locations in the city.
Those who get vaccinated at select subway stations can receive a free seven-day MetroCard. Anyone traveling on the Long Island Rail Road or Metro-North can get a free roundtrip ticket.
The goal of the initiative is to vaccinate 300 people a day.
AstraZeneca, J&J vaccines: summary continued
Regions using J&J vaccine with or without restrictions
EUROPEAN UNION
J&J said on April 20 it will resume rolling out its covid-19 vaccine in Europe with a warning on its label, after requesting countries, including Belgium, to pause the rollout.
FRANCE
Government said on April 21 that it plans to start using the vaccine the following week.
GERMANY
Is to make the J&J vaccine available to all adults, Health Minister Jens Spahn said on May 10.
GREECE
Plans to start rollout on May 5 after suspending vaccinations on April 19.
ITALY
Health ministry on April 20 recommended the vaccine be used for people over the age of 60.
NETHERLANDS
Resumed use of the vaccine from April 21.
POLAND
Started administering the J&J shot on April 15
SPAIN
Approved on Tuesday the use of the vaccine for people under the age of 60, Spanish El Pais newspaper reported. Spanish regions began using the vaccine to inoculate 70-79 year olds on April 22.
SWEDEN
Extended pause on J&J vaccine on April 23, adding that the shots could be given to people aged 65 and above.
UNITED STATES
Resumed use on April 23, ending a 10-day pause to investigate possible link to extremely rare but potentially deadly blood clots.
Regions where suspensions continue on J&J vaccine
DENMARK
Denmark on May 3 became the first country to exclude the shots from its vaccination programme.
AstraZeneca, J&J vaccines: summary continued
Regions where AstraZeneca vaccine use is suspended
CAMEROON
Said on March 18 it was suspending administration of shots the country was due to receive.
DENMARK
Said on April 14 it would stop using the AstraZeneca vaccine, the first country to do so. On April 19, Ritzau news agency reported that authorities may permit people to choose to have the vaccine.
NORWAY
A government-appointed commission said on Monday Norway should exclude vaccines made by AstraZeneca and J&J from its inoculation programme. Had suspended administration of AstraZeneca's shot on March 11.
AstraZeneca, J&J covid vaccines: summary
Following is an outline from Reuters of countries that have restricted or suspended use of covid-19 vaccines from AstraZeneca and Johnson & Johnson, after Europe confirmed possible links to rare blood clots.
J&J and AstraZeneca have stated that no clear causal relationship has been established between the clots and their vaccines. The European Medicines Agency (EMA) has so far maintained that the benefits of both the shots outweigh any risks.
The EMA is also reviewing reports of a rare nerve-degenerating disorder in people who received the AstraZeneca shot. First up...
Regions using AstraZeneca vaccine with restrictions
AUSTRALIA
Recommended on April 8 that people under 50 should get Pfizer's covid-19 vaccine in preference to AstraZeneca's.
BRAZIL
Brazil suspended use in pregnant women nationally on May 11 after an expectant mother in Rio de Janeiro died.
BRITAIN
Officials said on May 7 people under 40 should be offered an alternative to the vaccine where possible, lifting the age limit by 10 years from Britain's previous recommendation.
BULGARIA
Suspended use of vaccine on April 19 for women below 60 years who are at increased risk of thrombosis.
CANADA
Said in early April it would pause offering the vaccine to people under 55. Several provinces are now offering the AstraZeneca vaccine to people aged 40 and over.
ESTONIA
Suspended use for people under 60 on April 7.
FRANCE
Is using vaccine only for people aged 55 and over. On April 9, recommended that people under 55 who have had a first dose of the AstraZeneca shot should receive a messenger RNA vaccine for their second dose.
FINLAND
Is using only for people aged 65 and over.
GEORGIA
Is using only in medical centres Russian news agency TASS reported on March 19.
GERMANY
Germany said on May 7 it would give the shot to all adults who want it. It previously restricted use to those aged over 60 and recommended a different second dose for those under 60.
INDONESIA
Is using the vaccine but has warned against giving it to people with a low blood platelet count.
IRELAND
The government agreed to allow use of AstraZeneca and J&J vaccines for people over 50 years old, Prime Minister Micheal
Martin said on April 27. Had previously restricted use of AstraZeneca's vaccine to those over 60.
ITALY
Recommends use only for people over 60.
MALAYSIA
Began a parallel rollout last week for people to choose to receive the jab on a first-come, first-serve basis.
MEXICO
Drug regulator said on April 7 it did not "at this time" plan to limit the vaccine's use but was investigating the information raised by Britain.
NETHERLANDS
Said on April 8 it would limit use of the vaccine to people over 60.
NORTH MACEDONIA
Health minister said on March 31 the vaccine would be limited to people aged over 60 as a precautionary measure.
ONTARIO
The Canadian province said on May 11 it will stop offering first doses because of evidence that the risk of rare blood clots is somewhat higher than previously estimated.
PHILIPPINES
Said on April 19 it would resume administering the vaccine to under-60s after having temporarily suspended use on April 8.
SLOVAKIA
Health Ministry said on Tuesday it was suspending use for people getting their first doses, after experts reviewed the death of a recipient.
SOUTH KOREA
Resumed use of the shot for people aged 30 or older on April 12 after suspending use in under-60s on April 7.
SPAIN
Government said on April 30 it was extending the gap between the first and second doses of the vaccine to 16 weeks for people aged under 60. From April 8, Spain was giving the vaccine only to those over 60.
SWEDEN
Using for people aged 65 and older, while Swedes under 65 will be given an alternative to the AstraZeneca vaccine for their second dose.
Vaccine shortage in South Korea ahead of summit
South Korea’s struggle to boost coronavirus vaccine supplies is threatening to overshadow President Moon Jae-in’s first summit with US President Joe Biden, with pressure mounting on Moon to secure more and faster deliveries of US-made shots, report Reuters.
Moon had hoped to use the Washington meeting next week as a chance to highlight South Korea's relatively successful response to the pandemic, a key legacy in his final year in office.
But uncertainties in the country's vaccine rollout amid global shortages and shipment delays are deepening public scepticism over Seoul's goal of reaching herd immunity by November.
That has sparked calls for a deal to get faster access to vaccines from the United States and potentially sidelined other important issues for Moon and Biden such as North Korea policy or relations with China and Japan.
Full story below.
DEBUNKED: covid-19 vaccination myths for kids
The Food and Drug Administration on Monday authorized use of the Pfizer covid-19 vaccine for children in the US who are between 12 and 15 years olds, a step that infectious disease and public health experts believe is crucial to help the country return to some level of “normality.” It’s also exciting news for many parents who are eager to get their children vaccinated, especially after grappling with how to proceed when they are fully immunized but their kids are not.
But plenty of other parents are hesitant.
With all the covid-19 vaccine misinformation circulating, it’s understandable that some parents have concerns, and why, after a difficult year of pandemic parenting and so many unknowns, they’re wary of signing up their children for something they’ve been incorrectly told could be harmful.
Covid-19 vaccine for tourists in New York: how much and where can people get it?
New York City is offering US tourists a free souvenir to take home with them, a jab of the one-dose Johnson & Johnson covid-19 vaccine at iconic sites.
The city will let out-of-towners visiting get a covid-19 shot in an effort to draw more tourists to boost the economy and give local residents peace of mind.
Pfizer asks UK regulator to approve vaccine for teenagers
Pfizer Inc has formally asked the UK medical regulator for permission to use its Covid-19 vaccine for 12-to 15-year olds in Britain, the Telegraph reported on Tuesday. "We can confirm that the companies have submitted a request to the MHRA to expand the use of the Pfizer/BioNTech Covid vaccine in the UK to adolescents", the report said, citing a Pfizer spokesman.
Pfizer and the MHRA did not immediately response to Reuters requests for comment. The move comes as US regulators on Monday authorized Pfizer and BioNTech's Covid-19 vaccine for use in children as young as 12, widening the country's inoculation program as vaccination rates have slowed significantly.
Ontario will pause first doses of AstraZeneca vaccine
The Canadian province of Ontario will stop offering first doses of AstraZeneca's Covid-19 vaccine because of evidence that the risk of rare blood clots is somewhat higher than previously estimated, provincial officials said on Tuesday.
"This decision was made out of an abundance of caution due to an observed increase in the rare blood clotting known as vaccine-induced immune thrombotic thrombocytopenia," said Chief Medical Officer David Williams. "We are reviewing the data to consider options for the use of AstraZeneca for second doses and more broadly moving forward."
Vaccine urgency: India's covid deaths cross quarter million mark
India said on Wednesday a record number of people were killed by the coronavirus in the past 24 hours, pushing its overall death toll over a quarter million, while a leading virologist said it was too early to say if infections had reached a peak.
Deaths from covid-19 swelled by 4,205, while daily coronavirus cases rose by 348,421, with India's overall number of cases surging past 23 million, according to health ministry data. Even then, experts believe the official numbers grossly underestimate the real scale of the epidemic's impact, and actual deaths and infections could be five to ten times higher.
India's covid-19 infection curve may be showing early signs of flattening, but the decline in the number of new infections is likely to be slow, said Shahid Jameel, a top Indian virologist.
"It is still too early to say whether we have reached the peak," he was quoted as saying by the Indian Express newspaper.
"There is some indication of the cases plateauing. But we must not forget that this is a very high plateau. We seem to be plateauing around 400,000 cases a day."
Vaccine hesistancy challenge
Seatle Times reports on vaccine hesitancy after the results of a Poll by the Associated Press and NORC Center for Public Affairs Research were released.
"Just 11% of people who remain unvaccinated say they definitely will get the shot, while 34% say they definitely won’t, according to the poll by The Associated Press-NORC Center for Public Affairs Research."
Read our coverage on vaccine hesitancy here.
Why do vaccines make you feel ill?
All available vaccines have side effects and in most cases, they are completely normal; these side effects can even indicate that the vaccine is working.
The US Center for Disease Control and Prevention (CDC), has listed the possible side effects on their website and include: Tiredness , Headache , Muscle pain , Chills , Fever , Nausea
One other common symptom includes soreness or redness at the injection site. If these symptoms arise the CDC recommends that you speak with your primary care provider to talk about “taking over-the-counter medicine, such as ibuprofen, acetaminophen, aspirin, or antihistamines, for any pain and discomfort you may experience after getting vaccinated.”
Biden's vaccine objective
A reminder about yesterday's tweet from White House reporter, Kellan Howell:
"President Biden will announce today new steps to reach his goal of getting 70% of adult Americans vaccinated by July 4, including free Uber and Lyft rides to vaccine sites and on-sight clinics at community colleges across the country."
Vaccine hearing: Fauci and others speak
Watch top public health officials and experts in the US testify before the Senate Committee on Health, Education, Labor and Pensions on the status of our covid-19 response.

Over 35% of US population now fully vaccinated
At the time of reporting, a total of 116,576,359 Americans have received both doses of the covid-19 vaccine. That represents 35.1% of the population.
153,448,316 people, or 46.2%, have received at least one dose.
Track US covid-19 rollout data via the CDC
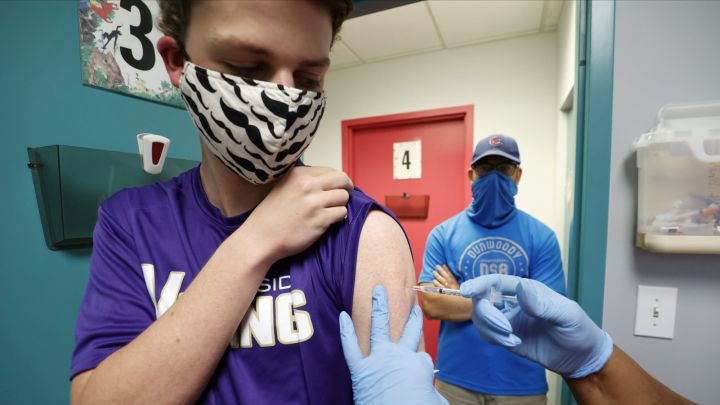
Photo: a teenager is inoculated with Pfizer's vaccine in Georgia on 11 May after state authorized it for ages over 12 years (Reuters/Chris Aluka Berry)
Covid-19 vaccine news: welcome
Hello and welcome to our dedicated live blog for Wednesday 12 May 2021.
Here we aim to keep you fully up to date with all the latest news and updates regarding the covid-19 pandemic and all aspects of the vaccine development and rollout across the United States and around the world.
Stay tuned...
- Coronavirus Covid-19
- Economic crisis
- Vaccines
- Pandemic
- Coronavirus
- Recession
- Pharmacy
- Economic climate
- Virology
- Outbreak
- Joseph Biden
- Infectious diseases
- Vaccination
- Microbiology
- Diseases
- Preventive medicine
- Medicine
- Enterprises
- Economy
- Biology
- Health
- Pfizer
- Industry
- Life sciences
- Moderna
- Johnson & Johnson
- AstraZeneca
- Covid-19 economic crisis
- Science
- Pharmaceutical industry
- Blood diseases